NASPGHAN recommends
ruling out metabolic disorders (such as LAL-D), medications, dietary causes, and infections when evaluating children for NAFLD3
All patient images are hypothetical

ruling out metabolic disorders (such as LAL-D), medications, dietary causes, and infections when evaluating children for NAFLD3

ruling out alternative causes of microvesicular steatosis like LAL-D when evaluating adults for NAFLD4
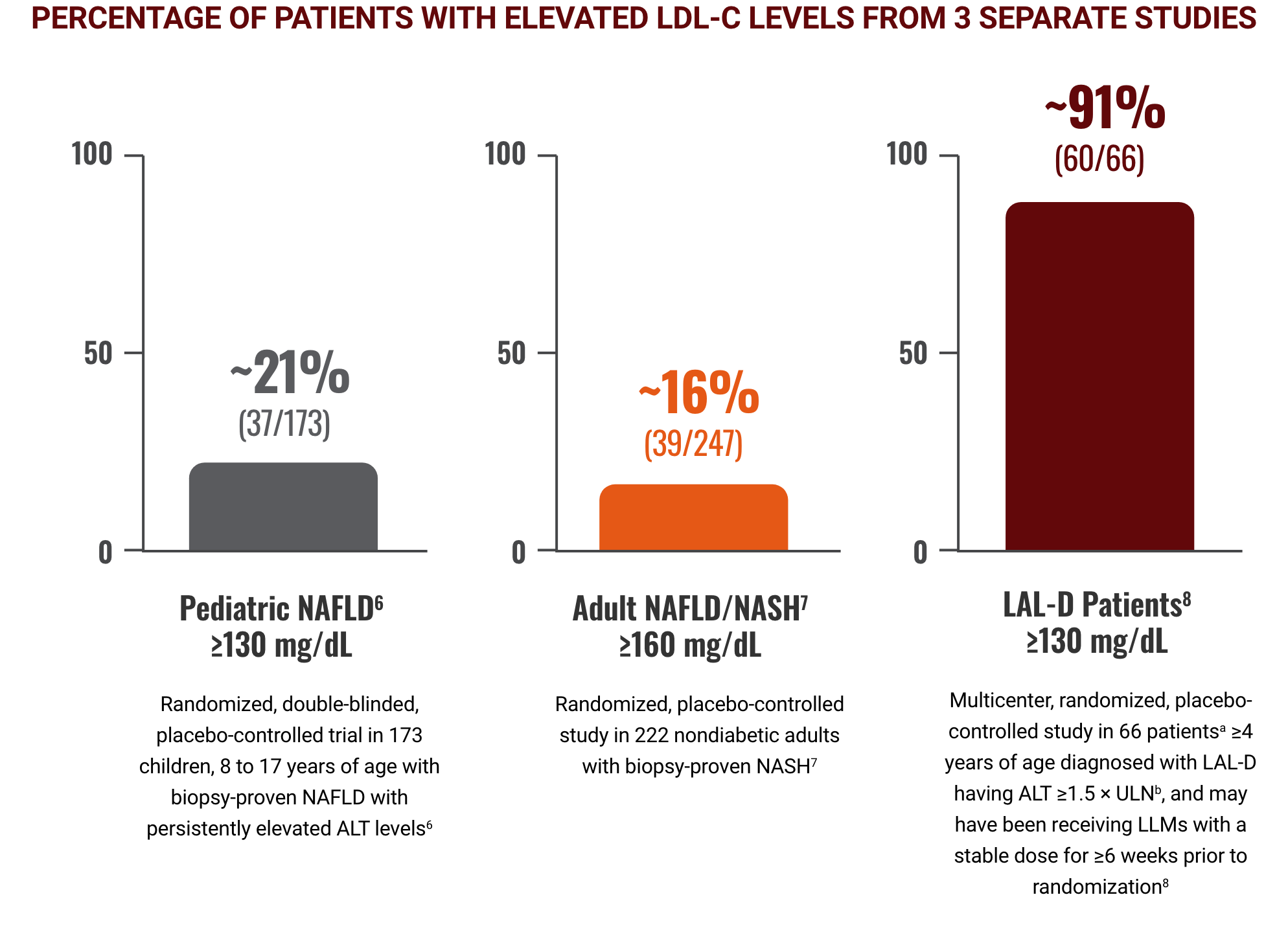
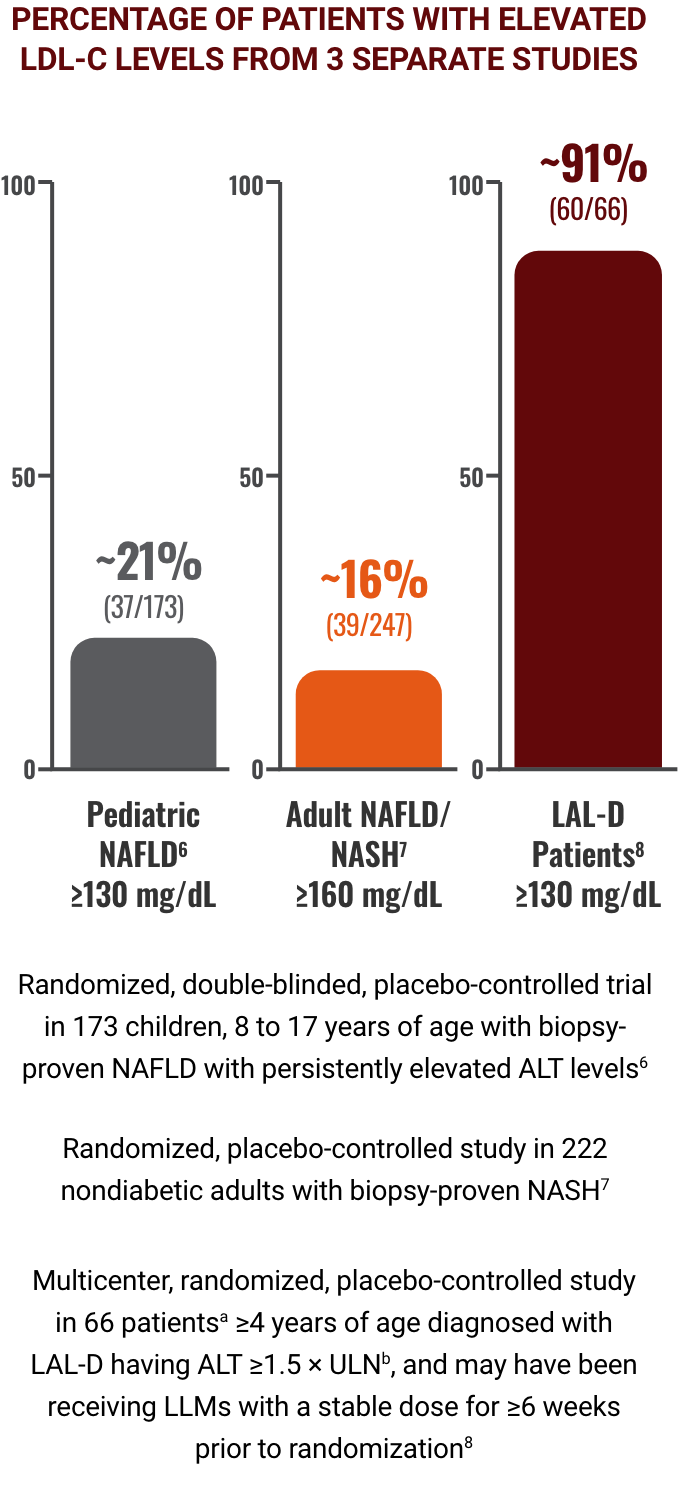
Image adapted from Corey KE, et al. J Pediatr Gastroenterol Nutr. 2015;60(3):360-367 and
Corey KE, et al. Aliment Pharmacol Ther. 2015;41(3):301-309
Percentages represent baseline characteristics of three separate studies in different patient populations; direct comparisons and implications of different management strategies cannot be made.
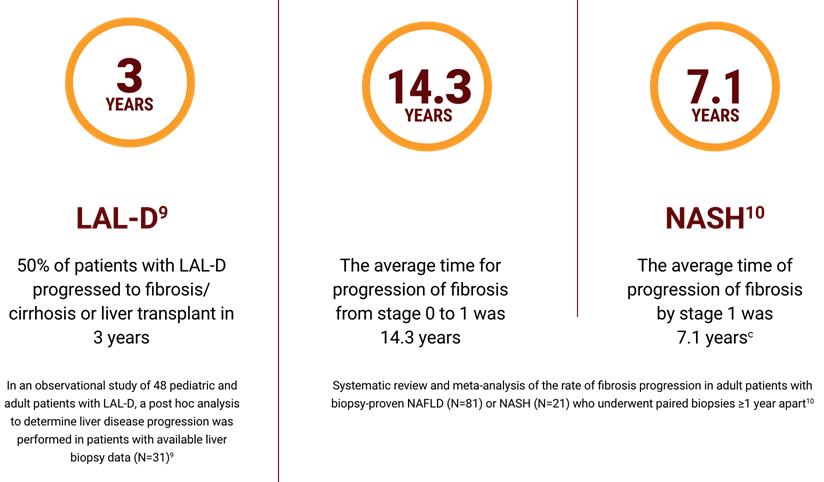

Note: Separate studies and distinct patient populations are shown; direct comparisons cannot be made.
NOTE: These studies represent individual publications using different patient populations and follow-up time. Data are not directly comparable.
a95% CI, 9.1-50.0 years.
bMost of the histologic assessment of fibrosis staging was done using Brunt’s classification. In Brunt’s classification: score 0 is the absence of fibrosis, score 1 is perisinusoidal/pericellular fibrosis, score 2 is periportal fibrosis, score 3 is bridging fibrosis, score 4 is cirrhosis.11
c95% CI, 4.8-14.3 years.
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; LAL-D, lysosomal acid lipase deficiency; LDL-C, low-density lipoprotein cholesterol; LLMs, lipid-lowering medications; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NASPGHAN, North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
References: 1. Reiner Ž, et al. Atherosclerosis. 2014;235(1):21-30. 2. Manns MP, et al. J Hepatol. 2015;62(1 suppl):S100-S111. 3. Vos MB, et al. J Pediatr Gastroenterol Nutr. 2017;64(2):319-334. 4. Chalasani N, et al. Hepatology. 2018;67(1):328-357. 5. Burton BK, et al. J Pediatr Gastroenterol Nutr. 2015;61(6):619-625. 6. Corey KE, et al. J Pediatr Gastroenterol Nutr. 2015;60(3):360-367. 7. Corey KE, et al. Aliment Pharmacol Ther. 2015;41(3):301-309. 8. Data on File, Alexion Pharmaceuticals. 9. Burton BK, et al. Curr Med Res Opin. 2017;33(7):1211-1214. 10. Singh S, et. al. Clin Gastroenterol Hepatol. 2015;13(4):643-654.e1-e9. 11. Santiago-Rolón A, et al. P R Health Sci J. 2015;34(4):189-194. 12. Lukacs Z, et al. Clinica Chimica Acta. 2017;471:201-205.
*All fields are required
By clicking Submit, I agree to the Terms of Use